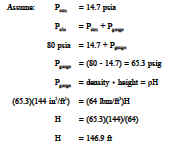Thermodynamic Pressure Scales Thermodynamics
Thermodynamic Pressure Scales
When pressure is measured relative to aperfect vacuum, it is called absolute pressure (psia) when measured relative to atmospheric pressure (14.7 psi), it is called gaugepressure (psig). The latter pressure scale was developed because almost all pressure gauges register zero when open to the atmosphere. Therefore, pressure gauges measure thedifference between the pressure of the fluid to which they are connected and that of the surrounding air. Ifthe pressure is below that of the atmosphere, it is designated as a vacuum.
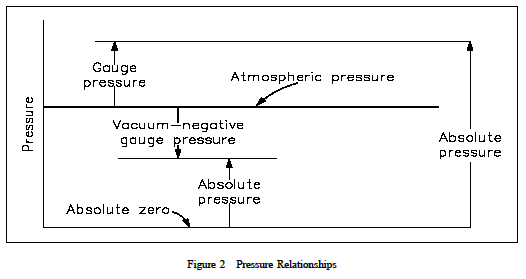
A perfect vacuum would correspond to absolute zero pressure. All values of absolute pressure are positive, because a negative value would indicate tension, which is considered impossible in any fluid. Gauge pressures are positive if they are above atmospheric pressure and negative if they are below atmospheric pressure. Figure 2 shows the relationships between absolute, gauge, vacuum, and atmospheric pressures, as do Equations 1-9 and 1-10.
![]()
P atm is atmospheric pressure, which is also called the barometric pressure. P gauge is the gauge pressure, and P vac is vacuum. Once again, the following examples relating the various pressures will be helpful in understanding the idea of gauge versus absolute pressures.
Example 1: Pressure Relationships
How deep can a diver descend in ocean water (density = 64 lbm/ft 3 ) without damaging his watch, which will withstand an absolute pressure of 80 psia? (P = density height)
Solution: