Temperature Entropy (T-s) Diagram - Thermodynamics
Thermodynamics Directory
Heat Transfer Directory
Temperature Entropy (T-s) Diagram
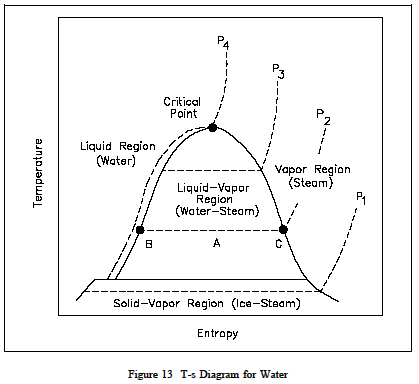
A T-s diagram is the type of diagram most frequently used to analyze energy transfer system cycles. This is because the work done by or on the system and the heat added to or removed from the system can be visualized on the T-s diagram. By the definition of entropy, the heat transferred to or from a system equals the area under the T-s curve of the process. Figure 1 is the T-s diagram for pure water. A T-s diagram can be constructed for any pure substance. It exhibits the same features as P-u diagrams.
Temperature Entropy (T-s) Diagram
In the liquid-vapor region in Figure 1, water and steam exist together. For example, at point A, water with an entropy (sf) given by point B, exists together with steam with an entropy (sg) given by point C. The quality of the mixture at any point in the liquid-vapor region can be found using the following relationship.
where
s = specific entropy of the mixture (Btu/lbm-°R)
x = quality of the mixture (no units)
sg = specific entropy of the saturated vapor (Btu/lbm-°R)
sf = specific entropy of the saturated liquid (Btu/lbm-°R)
sfg = specific entropy change of vaporization (Btu/lbm-°R) or sfg = sg - sf
Related
- Entropy Explained, With Sheep
- Closed System Cycles Thermodynamics Carnot Cycle & Entropy Class 10
- Entropy Definition and Equation
- Entropy Thermodynamics
- Fundamentals of Thermodynamics
- Enthalpy and Entropy of Saturated R-410a
- Water Saturation Properties Energy, Enthalpy and Entropy Table
- Enthalpy Entropy (h-s) or Mollier Diagram
- Thermodynamic Applications