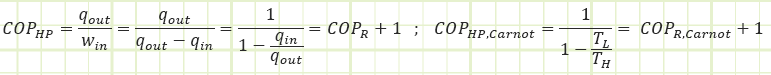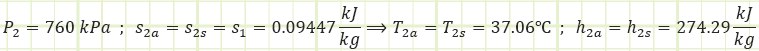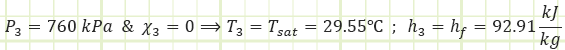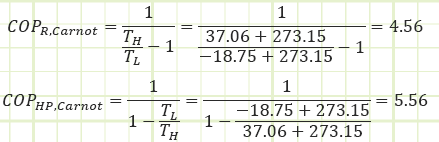Related Resources: thermodynamics
Steady Flow Vapor-Compression Refrigeration Cycle - Class 14
Thermodynamics Data, Equations, Charts, Equations and Calculators
Steady Flow Vapor-Compression Refrigeration Cycle - Class 14
Objective(s):
At the completion of the lecture, students should:
1) Identify the reference states, processes, and associated energy interactions of the Vapor-Compression Refrigeration Cycle.
2) Analyze a multiphase steady flow system using the Vapor-Compression Refrigeration Cycle with non-unity isentropic efficiencies.
3) Understand and use Coefficient of Performance as related to refrigeration and heat pump devices.
4) Observe applications of phase change phenomena applied to Vapor-Compression Refrigeration Cycle through examples.
Methodology:
In-class demo of a mini-fridge skeleton. Lecture with video (Inner workings for a refrigerator) shown via projector. A good resource to aid understanding of throttling in a capillary tube.
Terminology:
Refrigerant: A substance with desirable thermophysical characteristics; i.e. has a very cold saturation temperature for a moderately low pressure.
Throttling Device: An isenthalpic device that involves no heat or work interactions; it uses the Joule-Thomson effect to cause a decrease in temperature associated with a metered decrease in pressure. Non-reversible (i.e. non-isentropic).
Heat Pump: A device that uses mechanical work in order to make heat flow from a source at lower temperature to a sink at higher temperature. The desired output is the heat shed to the high temperature reservoir.
Refrigerator: A device that uses mechanical work in order to make heat flow from a source at lower temperature to a sink at higher temperature. The desired quantity is the heat taken from the low temperature reservoir.
Key Ideas:
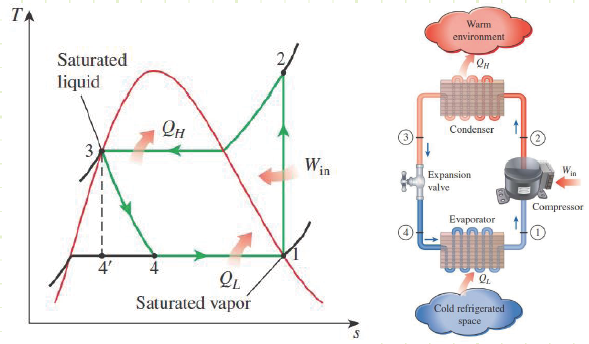
Ideal Vapor-Compression Refrigeration Cycle:
The most common method for relatively small temperature difference refrigeration (i.e. domestic cooling and heating)
Usually uses HFC-134a, but can also use R-152A, butane, propane, ammonia, even water in some cases, etc.
Cycle consists of four processes:
1) Isentropic compression in a compressor (State 1 to State 2). The substance is taken in as a saturated vapor at a lower pressure and pressurized isentropically in a compressor to a higher pressure. The work input is:
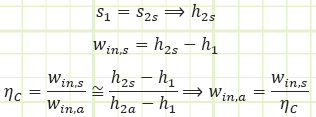
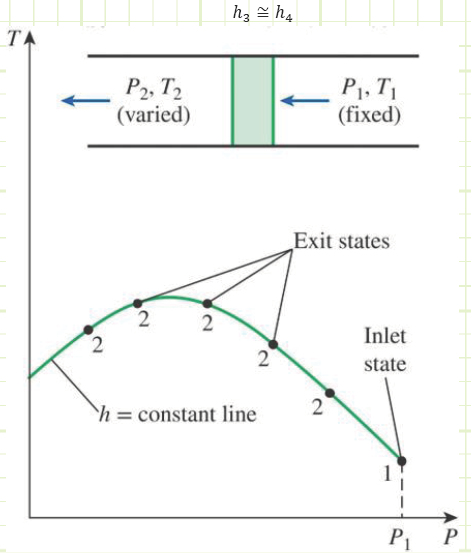
Special Note: ηc may actually turn out to be greater than 1! How? Some cooling may occur in the compressor while it does its thing. This is actually desirable, as this means it won’t have to work as hard to compress the refrigerant.
2) Isobaric sensible and latent cooling in a condenser (State 2 to State 3). Heat is rejected to a reservoir at elevated temperature, the substance condenses until it becomes a saturated liquid. The heat loss is:
![]()
3) Isenthalpic expansion in a throttling device (State 3 to State 4). The substance expands to a lower pressure in a device design to convert internal energy into flow energy, keeping enthalpy the same between the states. This is called Joule-Thomson expansion.
w = 0 ; q = 0
4) Isobaric latent heating in an evaporator (State 4 to State 1). The substance at very low pressure and low temperature accepts heat from a temperature source at relatively low temperature until it once again becomes a saturated vapor.
qin = ql = h4 - h1
Overall cycle performance for a refrigerator:
Overall cycle performance for a heat pump:
Sometimes we enter a philosophical argument as to what technology defines an era of human development.
There are the Stone Age, Bronze Age, Iron Age, Space Age, etc. I would argue that our current era exists in the Artificial Refrigeration Age. Ever since we harnessed the ability to pump heat uphill, we’ve taken MUCH greater control over our food supply (its longevity, its quantity/quality, and its transportability). We’re now able to exist in much less hospitable environs (imagine living in Las Vegas in a house WITHOUT A/C!!). We can build and use much more powerful supercomputers and advanced medical equipment. All of this and more because we can quickly and efficiently remove heat from a cool place and reject it to warmer surroundings. Essentially, this may be the cleverest thing our species has ever, and will ever, come up with…except for the long term side effects…
How does the Joule-Thomson effect even work? Well, I have a theory…
It only works with real substances. Ideal gases do not experience a change in temperature during an isenthalpic throttling process (why?). When real molecules are close together, their intermolecular forces have a pronounced effect. There’s actually a small sweet spot of minimal potential energy (due to interplay of electrostatic and Van Der Waals forces). When a collection of molecules is under high pressure and the average spacing between them is sitting in and around the sweet spot, it’ll consume internal energy to bring them apart. But when they’re throttled to a lower pressure, this is what happens. They expand away from each other via collisions, essentially doing internal work on each other and trading some of their chaotic internal kinetic energy (i.e. thermal internal energy) for larger potential energy as a result of being spaced farther apart from each other at lower pressure. This results in a lower temperature.
Ideal gases don’t have Van Der Waals forces. They interact ONLY via fully-elastic collisions. There is no potential energy curve with a sweet spot…It’s more like the shape of a capital L. No forces at any non-zero separation distance and then suddenly infinite repulsive force at zero separation distance.
The book discusses other more sophisticated mechanisms of refrigeration, such as the reversed Brayton cycle, which can achieve cryogenic temperatures, and cascading vapor-compression cycles where we stack one cycle on top of another, so the condenser of one reefer unit is the evaporator of the other. Sometimes we don’t even need an indirect heat exchanger and a mixing chamber (i.e. flash chamber) can be used between the cascading cycles. For liquefying gases, the Linde-Hampson cycle is pretty nifty. The absorption system uses ammonia and water and only needs a source of heat and a small pump to work. Finally, sound waves can actually be used to pump heat in a thermoacoustic refrigerator. These sound waves can actually be generated by heat itself.
EXAMPLE 1 – IDEAL VAPOR-COMPRESSION REFRIGERATION CYCLE.
Problem statement:
A refrigerator using R-134a flowing at 0.06 kg/s. Determine its performance characteristics
Given:
Known State Properties for Ideal Cycle:
Cycle Refrigerant (R-134a) flow:
![]()
Find:
a) Compressor work, heat rejected from the condenser & heat removed from cold reservoir. b) Isentropic efficiency of the compressor.
c) COPR and COPHP
Assumptions:
1) Vapor-compression refrigeration cycle.
2) Process 3 → 4 is isenthalpic.
3) Excel Thermotables Add-In from University of Alabama is accurate. For tabular interpolation treatment, see example 2. Some differences between what’s in the textbook appendices and the values you see here may exist; they should be small and not really affect the overall analysis.
Diagram(s):
Solution:
Determine properties at important stages in the cycle:
Properties at State 1 (Saturated Vapor):
Process 1 → 2s and 1 → 2a (Isentropic and actual compression in a compressor):
Comparing these properties to those in Table A-12 (looking at Tsat for both 750 & 800 kPa), it is determined the refrigerant is not in a saturated state. The temperature is too high; therefore, it is a Superheated Vapor.
Properties at State 3 (Saturated Liquid):
Process 2a → 3 (Isobaric heat rejection from a condenser):
Process 3 → 4 (Isenthalpic throttling in a thermal expansion device):
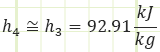
Properties at State 4 (Saturated Mixture):
Process 4 → 1 (Isobaric heat absorption by an evaporator):
Cycle Performance Metrics:
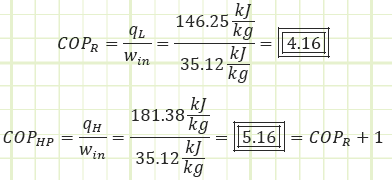
A device operating on the reversed Carnot cycle would operate between the maximum and minimum temperature differences with the following COPs:
And we find our ideal cycle is actually very close to the fully reversible performance (best thermodynamically possible). A quick inspection of the T-s diagram tells us why; the shape of our internally-reversible cycle is very close to a perfect rectangle (i.e. two isothermal and two isentropic processes), which is characteristic of Carnot. Had we compressed to a higher pressure, the departure from Carnot would be more pronounced. In fact, a condenser temperature of 37.06°C to 29.55°C probably isn’t high enough for sufficient heat transfer to occur unless the condenser was very large with a lot of surface area in a relatively colder environment than is typically seen in a house.
Source
Unknown Contributor - Reddit
Related:
- Introduction to Thermodynamics, Class 1
- Ideal Gas Assumptions, Properties of Pure Substances, Property Tables, Class 2
- Control Volume Analysis, Reynolds Transport Theorem, Conservation of Mass, and the First, Class 3
- Mechanical Work for Closed Systems Class 4
- Properties of Pure Substances , Phase Changes Class 5
- Thermodynamics of Multiphase Closed Systems Class 6
- Analysis of Open Systems Thermodynamics Class 7
- Introduction to Otto Cycle, Cycle Thermal Efficiency, Spark-Ignition Engine Architecture, and Combustion Cycle Class 8
- Closed System Cycles Ideal & Real Diesel Cycle Class 9
- Closed System Cycles Thermodynamics – Carnot Cycle & Entropy Class 10
- Steady Flow Gas Power Cycles – Brayton Cycle Class 11
- Steady Flow Vapor Power Cycles Thermodynamics – Rankine Cycle - Class 12
- Steady Flow Vapor Power Cycle – Rankine Cycle w/ Open Feedwater Heating - Class 13
- FIRST LAW OF THERMODYNAMICS
- SECOND LAW OF THERMODYNAMICS
- Thermodynamic Systems and Surroundings
- Types of Thermodynamic Systems
- Thermodynamic Equilibrium
- Control Volume
- Steady State
- Thermodynamic Process
- Cyclic Process
- Reversible Process
- Irreversible Process
- Adiabatic Process
- Isentropic Process
- Polytropic Process
- Throttling Process