Battery Negative and Positive Plate Construction
Battery Application & Technology
The simplest method for the construction of lead-acid battery electrodes is the plant plate, named after the inventor of the lead-acid battery. A plant plate is merely a flat plate composed of pure lead. Since the capacity of a lead-acid battery is proportional to the surface area of the electrodes that is exposed to the electrolyte, various schemes are employed to increase the surface area of the electrodes per unit volume or weight. Plant plates are grooved or perforated to increase their surface area. A typical plant plate is shown below.
Plate
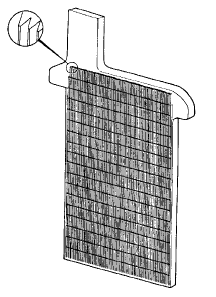
The most commonly used method to increase surface area is to make the active material into a paste that acts like a sponge where the electrolyte fills all the pores. The paste, or active material, is mounted into a frame or grid structure that mechanically supports it and serves as the electrical conductor carrying the current during both the charge and discharge cycle. The most commonly used plate today is the pasted plate, also known as the flat plate. This grid structure is a lattice-work that resembles the cross section of a honeycomb, with the paste filling all of the rectangular windows on the structure. The picture below shows a typical construction of a pasted plate grid. The flat plate construction is used as the negative electrode plate in almost all cases, and serves as the positive plate in most standby applications.

Pasted Grid plate
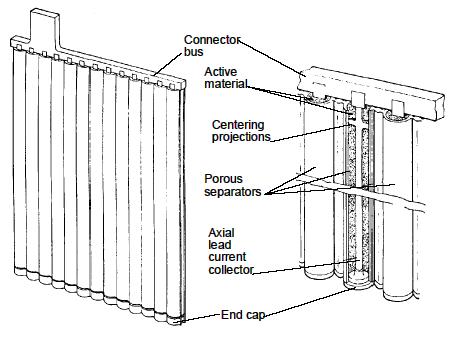
Positive electrodes are usually of pasted plate or tubular construction. Tubular electrodes are popular positive plates for heavy cycling applications. This construction uses a frame structure consisting of a series of vertical spines connected to a common bus. The paste is held in micro-porous, non-conductive tubes which are placed over the individual spines. A simplified view of tubular plate construction is shown in below. Regardless of the plate type used, the capacity of any battery is increased by adding multiple plates in parallel.