Critical Point Thermodynamics - Thermodynamics
Thermodynamics Directory | Heat Transfer Directory
Critical Point Thermodynamics
In thermodynamics , a critical point (or critical state ) is the end point of a phase equilibrium curve. The most prominent example is the liquid-vapor critical point, the end point of the pressure-temperature curve that designates conditions under which a liquid and its vapor can coexist. At the critical point, defined by a critical temperature T c and a critical pressure p c , phase boundaries vanish. Other examples include the liquid–liquid critical points in mixtures.
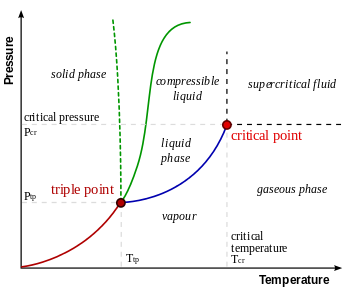
The Liquid-vapor critical point in a pressure–temperature phase diagram is at the high-temperature extreme of the liquid–gas phase boundary. The dotted green line shows the anomalous behavior of water.