Work - Thermodynamic Properties - Thermodynamics
Thermodynamics Directory | Heat Transfer Directory
Kinetic energy, potential energy, internal energy, and P-V energy are forms of energy that are properties of a system. Work is a form of energy, but it is energy in transit. Work is not a property of a system. Work is a process done by or on a system, but a system contains no work. This distinction between the forms of energy that are properties of a system and the forms of energy that are transferred to and from a system is important to the understanding of energy transfer systems.
Work is defined for mechanical systems as the action of a force on an object through a distance. It equals the product of the force (F) times the displacement (d).
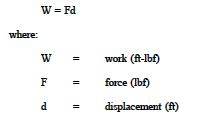
Example:
Determine the amount of work done if a force of 150 lbf is applied to an object until it has moved a distance of 30 feet.
Solution:
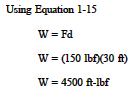
In dealing with work in relation to energy transfer systems, it is important to distinguish between work done by the system on its surroundings and work done on the system by its surroundings. Work is done by the system when it is used to turn a turbine and thereby generate electricity in a turbine-generator. Work is done on the system when a pump is used to move the working fluid from one location to another. A positive value for work indicates that work is done by the system on its surroundings; a negative value indicates that work is done on the system by its surroundings.