Related Resources: fluid flow
Handbook of Physical Constants Use in Atmospheric Studies
Handbook of Physical Constants and Functions for Use in Atmospheric Boundary Layer Studies
Edgar L Andreas, 2005
Cold Regions Research and Engineering Laboratory
U.S. Army Engineer Research and Development Center
54 pages
Free Membership Minimum Required to view Document/Book
Open: Handbook of Physical Constants and Functions for Use in Atmospheric Boundary Layer Studies
Introduction
Studies of geophysical boundary layers always require kinematic and thermodynamic constants for the fluids involved. The most obvious examples are for the fluid densities: air, pure water, seawater, and water vapor. Boundary-layer studies frequently involve exchanging properties across interfaces; consequently, molecular properties like the kinematic viscosity, thermal conductivity, water vapor diffusivity, and surface tension are also necessary. In turn, the ratios of the molecular transport quantities—the Prandtl and Schmidt numbers—are recurring variables.
Here, I summarize the values and functions for these and other quantities that I have found to be accurate and useful for my work in boundary-layer meteorology. This handbook is admittedly not all-encompassing. For example, I tend to focus on the quantities and the range of conditions for studies of air–land, air– sea, and air–sea–ice interaction. I also describe some microphysical quantities used in studies of aqueous solution droplets, like cloud droplets and sea spray. I tend to simply describe ways to calculate the quantities of interest without also explaining why you might want this quantity.
TOC
LIST OF SYMBOLS v
PREFACE. ix
1 INTRODUCTION. 1
2 AIR DENSITY 2
3 WATER VAPOR DENSITY .. 3
4 WATER VAPOR VARIABLES 5
4.1 Vapor Pressure 5
4.2 Specific Humidity . 7
4.3 Mixing Ratio 8
4.4 Relative Humidity . 8
4.5 Wet-Bulb Temperature 9
5 WATER DENSITY 13
6 SPECIFIC HEAT 15
6.1 Specific Heat of Dry Air . 15
6.2 Specific Heat of Water Vapor .. 15
6.3 In the Turbulent Sensible Heat Flux.. 15
6.4 Specific Heat of Water . 17
6.5 Specific Heat of Ice 18
7 LATENT HEAT.. 20
8 SURFACE TENSION OF WATER.. 21
9 MEAN FREE PATHS OF AIR AND WATER VAPOR MOLECULES.. 23
10 MOLECULAR VISCOSITY 25
10.1 Air. 25
10.2 Water .. 25
11 THERMAL CONDUCTIVITY AND THERMAL DIFFUSIVITY
OF AIR 28
12 MOLECULAR DIFFUSIVITIES OF GASES IN AIR 29
12.1 Water Vapor 29
12.2 Other Atmospheric Gases. 29
13 EFFECTS OF SURFACE CURVATURE ON ka AND Dv .. 32
14 WIND FORCE SCALES 34
14.1 Beaufort Scale 34
14.2 Saffir-Simpson Scale.. 34
14.3 Fujita Scale.. 37
15 CONCLUSIONS. 38
REFERENCES . 39
ILLUSTRATIONS
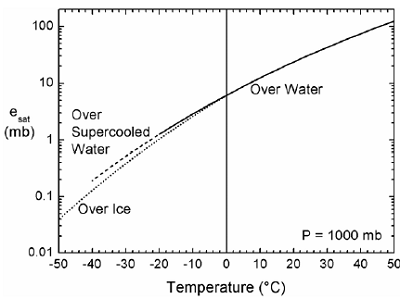
Figure 1. Saturation vapor pressure as a function of temperature
for three regimes: vapor over a water surface, vapor over
supercooled water, and vapor over ice . 6
Figure 2. Specific heats at constant pressure of dry air, water vapor,
pure water, and pure ice .19
Figure 3. Molecular values of the kinematic viscosity and thermal
diffusivity of air and of the water vapor diffusivity in air .26
Figure 4. Effects of surface curvature on the thermal conductivity of air
and on the water vapor diffusivity in air 33
TABLES
Table 1. Relative humidity as a function of the air temperature
and the dew-point depression .10
Table 2. Vapor pressure as a function of the air temperature
and the wet-bulb depression 12
Table 3. Specific heat of seawater at constant pressure as a function
of temperature and salinity ..18
Table 4. Molecular values of the kinematic viscosity and thermal
diffusivity of air, the diffusivity of water vapor in air,
and the Prandtl and Schmidt numbers 27
Table 5. Molecular diffusivities of various gases in air 31
Table 6. Beaufort Scale .35
Table 7. Saffir-Simpson Scale ..36
Table 8. Fujita Scale ..37