Related Resources: fluid flow
Osmotic Pressure Equation
Civil Engineering Application & Design Resources
Fluids Flow Design and Engineering
Osmotic Pressure Equation
Osmosis is a special case of diffusion in which molecules
of the solvent move under pressure from one fluid to
another (i.e., from the solvent to the solution) in one
direction only, usually through a semipermeable membrane. Osmosis continues until sufficient solvent has
passed through the membrane to make the activity (or
solvent pressure) of the solution equal to that of the
solvent.20 The pressure at equilibrium is known as the
osmotic pressure, π.
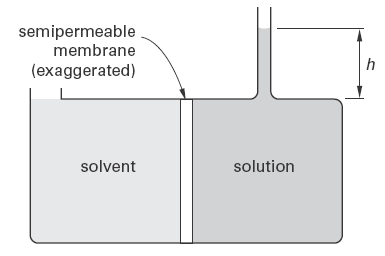
Figure 1 illustrates an osmotic pressure apparatus. The fluid column can be interpreted as the result of an osmotic pressure that has developed through diffusion into the solution. The fluid column will continue to increase in height until equilibrium is reached. Alternatively, the fluid column can be adjusted so that the solution pressure just equals the osmotic pressure that would develop otherwise, in order to prevent the flow of solvent. For the arrangement in Fig. 1, the osmotic pressure can be calculated from the difference in fluid level heights, h.
Eq. 1, SI
π = ρ · g · h
Eq. 2, U.S. π = ρ · g · h / gc
Where
π = osmotic pressure, atm
ρ = fluid density lbm/ft3, ( kg/m3 )
g = gravity, gravitational acceleration,
32.2, (9.81), ft/sec2 ( m/s2 )
h = difference in fluid
level height, (ft, m)
gc = gravitational constant, lbm-lbf/lbf-sec2
Figure 1
Osmotic Pressure Apparatus
Source:
Civil Engineering Reference Manual, Fourteenth Edition
Michael R. Lindeburg, PE
Related