Related Resources: thermodynamics
Properties of Pure Substances , Phase Changes Class 5
Thermodynamics Data, Equations, Charts, Equations and Calculators
Properties of Pure Substances, Phase Changes Class 5
Objective(s):
At the completion of the lecture, students should:
1) Understand the concept of latent heat as it relates to a change of phase.
2) Understand the concept of sensible heat as it relates to a temperature change.
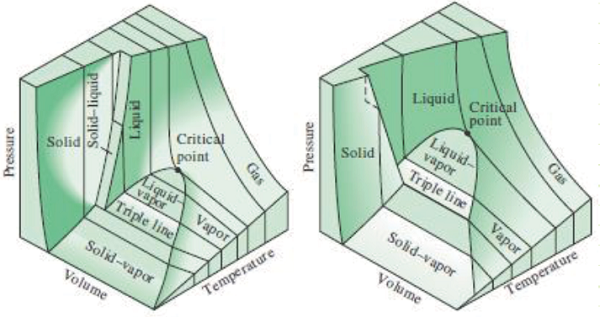
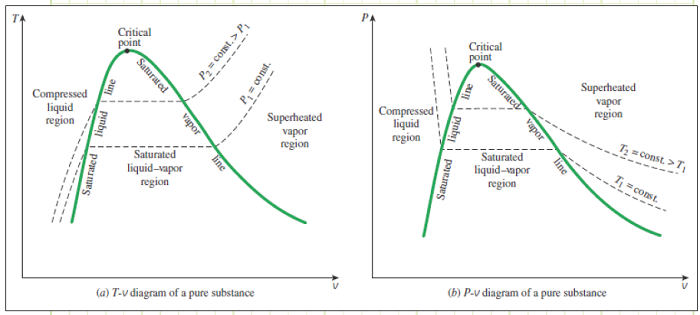
3) Understand how P-v and T-v diagrams depict properties of multiphase systems.
4) Understand and apply the property of Quality with regard to saturated liquid-vapor mixtures.
5) Analyze a multiphase closed system process.
Methodology:
Lecture with animations and video (Triple Point, Critical Point, Steam Power 1, Steam Power 2, Steam Turbines) shown via projector. Hero’s Turbine and Steam Engine.
Terminology:
Latent Heat: The amount of thermal energy required to cause a change in phase of a substance. Latent Heat of
Fusion is the energy required to cause melting (when heat is added) or freezing (when heat is extracted). Latent Heat of Vaporization is the energy required to cause vaporization (when heat is added) or condensation (when heat is extracted). Temperature does not change during a constant pressure phase change process.
Sensible Heat: The thermal energy associated with a change in temperature of a substance.
Saturation: When a substance is either about to undergo or is currently undergoing a phase change. At saturation, temperature and pressure are fixed and no longer independent from each other. Any addition of heat will cause further phase change and no change in temperature when pressure is constant as long as the two different phases continue to coexist.
Vapor pressure: The pressure required to keep a substance a liquid when held at a certain temperature. A decrease in pressure below the vapor pressure will allow vaporization to occur. If I have a cup of liquid water at sea level and its temperature is 76°C, then it’s not going to be boiling. If I instantly relocate my cup to the peak of Mount Everest where the atmospheric pressure is about one-third its sea level value, then the water will begin to spontaneously boil and change into vapor.
Compressed liquid: Also called “subcooled liquid,” is a liquid that is not about to vaporize; either its pressure must decrease or temperature must increase (or a combination of the two) in order to cause vaporization. Specific internal energy, specific enthalpy, specific entropy, and specific volume are not strong functions of pressure for a compressed liquid, and for moderately small pressures, these properties can be approximated as being equal to those of a saturated liquid having the same temperature, (i.e. hour liquid = hf of saturated liquid @ our liquid’s T).
Saturated liquid: A liquid that will begin vaporizing if any heat is added.
Saturated mixture: When a saturated liquid and saturated vapor coexist in the same system. Heat addition causes more vaporization. Heat removal causes more condensation.
Saturated vapor: A vapor that will begin condensing if any heat is removed.
Superheated vapor: A vapor that is not about to condense. Adding heat raises it temperature, removing heat lowers its temperature.
Critical point: The temperature and pressure at which a liquid becomes indistinguishable from a vapor. Above the critical temperature, no amount of pressure will force condensation to occur.
Quality: The ratio of saturated vapor mass against total mass of a substance in a system. Only a relevant property for a saturated mixture (inside the vapor dome). Ranges between 0 (100% of mass exists as a saturated liquid) and 1 (100% of mass is saturated vapor). The value of quality is undefined for compressed liquids and superheated vapors.
Key Ideas:
Phase Change:
Isobaric heating of a pure substance from frozen solid to superheated vapor:
Landmark Properties:
vf: Specific volume of a saturated liquid. vg: Specific volume of a saturated vapor.
vfg: vg - vf: The space between the saturated vapor and saturated liquid lines representing the net change in specific volume of a substance as it completely vaporizes from a saturated liquid state to a saturated vapor state. The foregoing also applies for u, h, and s.
χ: Quality. The ratio of saturated vapor mass against total mass of a substance in a system. Only a relevant property for a substance inside the vapor dome. Ranges between 0 (saturated liquid) and 1 (saturated vapor).
χ = mvapor / mtotal
Quality can be determined by knowledge of specific properties of your saturated mixture relative to the bounding properties of the vapor dome:
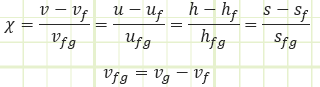
How do we use this information?
Appendices A-4 & A-5 display saturated mixture data for water. Appendix A-6 shows superheated steam. Appendix A-7 shows compressed liquid water. Similar appendices exist for R-134a or you can go to the NIST Chemistry WebBook to generate information tables for just about any common substance.
Sometimes, especially in the superheated vapor region, you’ll need to linearly interpolate between values.
EXAMPLE 1 – WATER IN A STEEL DRUM.
Problem statement:
Some yahoo throws a sealed steel drum containing only pure water on a fire and heats it up until the internal temperature is uniformly 150°C.
Given:
Properties at State (1):
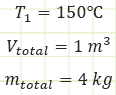
Find:
a) Mass of vapor.
b) Volume of vapor.
Assumptions:
1) Volume is constant and steel drum won’t explode or implode.
Diagram(s): 150°C

Solution:
Determine the actual specific volume of water in the drum:
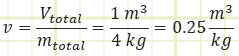
How does this value compare to the saturated liquid and vapor specific volume values for water at the specified temperature? If our specific volume occurs between vf and vg, then we know we’re under the saturation dome.
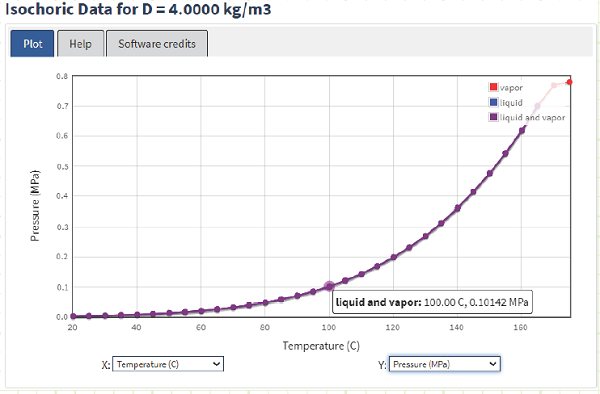
Using the NIST WebBook, we can quickly determine the pressure inside the drum:
On the T-v and P-v diagrams, we can plot our current state using NIST saturation data:
It’s also pretty easy to obtain Quality using NIST.
If we were to add more heat (or allow it to cool down), what kind of process is occurring? Let’s say we do allow it to cool down. What will happen to its absolute pressure? At what temperature is the internal pressure the same as the external pressure (i.e. 101.325 kPa(a))?
What will probably occur with further cooling? Maybe this, this, or this?
Source
Unknown Contributor - Reddit
Related:
- Introduction to Thermodynamics, Class 1
- Ideal Gas Assumptions, Properties of Pure Substances, Property Tables, Class 2
- Control Volume Analysis, Reynolds Transport Theorem, Conservation of Mass, and the First, Class 3
- Mechanical Work for Closed Systems Class 4
- FIRST LAW OF THERMODYNAMICS
- SECOND LAW OF THERMODYNAMICS
- Thermodynamic Systems and Surroundings
- Types of Thermodynamic Systems
- Thermodynamic Equilibrium
- Control Volume
- Steady State
- Thermodynamic Process
- Cyclic Process
- Reversible Process
- Irreversible Process
- Adiabatic Process
- Isentropic Process
- Polytropic Process
- Throttling Process